The Birth of a Male Contraceptive
An IIT professor has developed an original drug called Risug that simplifies vasectomy to a single injection. And it’s easily reversible too. The thing is, he has been waiting 33 years for the Government’s final approval of his wonder drug
 Sohini Chattopadhyay
Sohini Chattopadhyay
 Sohini Chattopadhyay
|
01 Apr, 2012
Sohini Chattopadhyay
|
01 Apr, 2012
/wp-content/uploads/2015/11/male-contra.jpg)
An IIT professor has been waiting 33 years for the Government’s final approval of his wonder drug
It was while purifying water that the thought of contraception came to the mind of Professor Sujoy Kumar Guha. The IIT-Delhi professor, who had already earned the reputation of a maverick, had been commissioned with finding a cheap, effective means to disinfect drinking water by the Government of India.
Guha, an admirer of the eel, had written a paper—as part of his PhD research—on how this fish generates formidable electric shocks to fob off predators, and sought a solution inspired by this defence mechanism. The idea was to generate a charge in water that would kill E. coli bacteria and other contaminants. Trained in electrical engineering and armed with BTech and MTech degrees from IIT-Kharagpur (where he now teaches) the professor initially tried using engineering instruments such as the Van de Graaf generator (of voltage). Somehow, despite sparks of promise, it didn’t work out.
Guha then thought of using a chemical compound to achieve the same result: killing the infecting organisms with an electrical charge. The challenge was to find something that could stay in water for several days, generate voltage, and yet leave the water fit for consumption. He worked out a solution, but the Health Ministry’s priorities had changed by then.
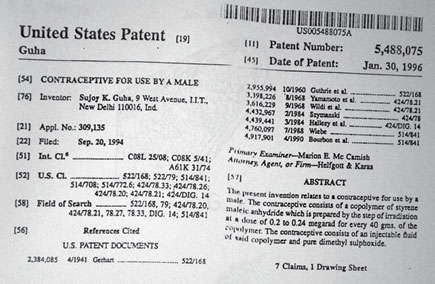
(The US patent that Guha got for his Risug contraception method)
Guha, however, a man curiously impervious to disappointment, was already on to a fresh idea: if diehard E. coli bacteria, with their tough hairy coverings, could be defused this way, then why not sperm? This idea, he thought, could be used to develop a new contraceptive for male use.
After some chemical ciphering, the professor formulated the original compound Styrene Maleic Acid Anhydride with Dimethyl Sulfoxide (SMA+DMSO). Injected into a male body, this dense compound forms a resolute polymer inside the vas deferens—the vein that carries sperm from the testes to the penis. Sperm are negatively charged, while the polymer has both negative and positive charge. The positive ions predominate, however, and neutralise the charge of the sperm. A positive charge cancelling out negative ions: this is the delightfully uncomplicated principle of Guha’s cool new contraceptive Risug, short for Reversible Inhibition of Sperm under Guidance.
In regular vasectomy, the vas deferens is severed and the loose ends are tied up—a far messier procedure, and one that is harder still to reverse. Risug is administered through only two injections, one on each scrotum’s side, and is reversed just as easily with a couple of injections to flush out the drug.
Most of that was worked out in 1975. Guha published a four-page paper in 1979, and began clinical trials on animals; first with rats (small animals), then with monkeys (large animals). He was 40 years old at the time. He is 72 now, and extended Phase III trials on humans are finally underway. In the 300-plus subjects who have undergone the Risug procedure so far, the drug has proved to be effective and entirely free of side effects (a frequent bugbear of old-style vasectomy that deters some men almost as much as the thought of a vein being cut).
Guha senses that he is close to getting approval for his drug, but knows he is not there yet. In the 33 years that he has been working to get the drug licensed, his wife has not seen him lose sleep, clumps of hair or his appetite. “He’s got upset at times,” says Mrs Rita Guha, “but then he tried to do something about the matter.” Indeed, the professor has even taken the Government to court. “These things take time,” he says calmly.
The professor’s efforts are being tracked across the world. The US-based Parsemus Foundation acquired technology-transfer rights from Guha in 2010 to produce Risug in the US. It is expected to move the US Federal Drug Authority for approval in a couple of years, and is likely to market it as Vasalgel. In 2011, the Paris-based World Academy for Biomedical Technologies asked permission from Guha to introduce the drug in France, Germany, Hungary, Italy, the UK and possibly Greece and Ukraine. The WABT and a clinical specialist in Germany are planning to begin clinical studies of an advanced version of Risug named Risugadv this year. Meanwhile, Guha’s Yahoo mail inbox bulges with entreaties from men in the West—the US and Israel mostly—who want to volunteer as Risug trial subjects. ‘Prof, I believe Risug might be my only chance to ever enjoy a normal relationship with women again. I beg you, please let me participate in one of the tests, formally or informally,’ implores an Israeli man, for example.
A fourth phase of monitored marketing of Risug is set to begin this year in a few districts of India. Yet, what remains impossible to predict is when the final clearance of the Drugs Controller General of India will come through. Dr Hem Das of Delhi’s Lok Nayak Hospital, who has been one of the chief clinical practitioners on Risug trials since 1995, gestures heavenward in response to the question. “Two years, ten years, who knows? I hope Professor Guha lives to see it through,” he says.
“One of the main reasons for the delay is that international protocol for the approval of new drugs has changed during these years,” says Dr RS Sharma, deputy director general, senior, of the Indian Council of Medical Research (ICMR). “The new guidelines required us to conduct new tests. This set us back by many years. But now, we’re nearly done with the testing,” he says, refusing to hazard a guess on when Risug will get an all-clear.
Ever the optimist, Guha believes that the drug could get approval in less than two years. “Risug could not have come so far without support from the Health Ministry and ICMR,” he says, “Three health secretaries in particular have backed me greatly.” Besides, a drug licence must necessarily take time, given the rigour of the process. Much has to be verified: non-toxicity, non-cancerous tendencies, and the possibility of side effects, reasons Guha, who has been a picture of resolute unflappability—if occasional pushiness—all these years.
More than once, the drug’s clinical trials have been stopped, stalling work for years together. But Guha managed to revive the project each time. In 1993, when Risug had just entered its second phase of clinical trials, somebody photocopied select pages of a book called Hazardous Chemicals: Desk Reference by N Irving Sax and Richard J Lewis Sr, and sent them to the Health Ministry. The pages chosen were on Styrene and Maleic Anhydride, which the book listed as ‘HR3 carcinogens’—the highest class of cancer-causing substances. Work stopped. Guha’s defence was simple and it settled the matter: individual substances may be carcinogenic, but the compound is not. Case in point: placed on your palm, pure sodium can melt the skin off your hand, as can pure chloride, but together, sodium chloride is not just better-tempered, it is palatable. We know it as salt.
When there was no sign of clinical trials resuming by 1996, Guha filed a legal case against the Government. He hired Siddharth Shankar Ray, legendary lawyer and former Chief Minister of West Bengal, and when the matter came up for hearing, the court took five minutes to reach a conclusion: this was not a matter for it to decide; the petitioner could either withdraw the case or have it dismissed by the Supreme Court. Ray withdrew. “The Supreme Court gave 40 minutes to a property matter in Connaught Place… but they didn’t have five minutes for me. Still, I think the Government got the message: that ‘this guy will not stop at anything’,” recounts Guha.
Trials resumed soon after, and Phase II was completed in 1997-98. Phase III trials began in 1999, and things went really well. So well that in 2002, the then Union Health Minister CP Thakur held a press conference at which he announced Risug, saying that it would likely be available for use within a year. But barely weeks later, Guha found the drug being cast as among the ‘most dangerous substances in the world’. A study had found traces of the protein albumin in urine samples of some Risug trial subjects. The project was called off on account of danger posed to the kidneys.
Guha says it’s common in India to find traces of albumin in urine samples of men, with or without Risug injections. This is typically so in summer. When he pointed this out, nothing happened. So in 2005, when things still hadn’t moved, he took along clear jars and testers to a high-level meeting. Just before the delegation was about to break for lunch, he asked the attendees to collect some of their urine in a jar each and hand it over to him after lunch. He wanted to test it for the presence of albumin. Plenty of people walked off in a huff and didn’t return. “I don’t think that achieved anything in concrete terms,” chuckles Guha, “But I had a point to prove.”
Trials resumed in 2007, and have not stopped since. Guha feels that many of the questions raised about Risug and the delays that have dogged the project have been at the instigation of the National Institutes of Health (NIH)
in the US. Dr Gulshanjit Singh, head of the surgery department at Vardhaman Mahavir Medical College and Safdarjung Hospital, says there was once a time when America’s NIH was trying to promote a hormone-based, repeat-use contraceptive made by a US-based company. Dr Singh, who was the first clinical practitioner to administer the drug, remembers meetings where a section of the ICMR argued strongly in favour of the American contraceptive and pointed out problems with Risug.
“Right from the beginning, there have been people within the ICMR who opposed Risug,” says AR Nanda, who was the Union health secretary from 1999 to 2002. “And the whole process was set back seven to eight years in 2002, when the toxicology issue came up. In essence, Guha was asked to prove again what he had already proven. I brought this up even in a WHO meeting in Geneva.” Nanda, a big supporter of Guha, has spoken out against the ICMR’s handling of trials on numerous occasions. “In fact, even in the WHO, there are people who don’t want the drug to come through.” At one point, the WHO had expressed interest in Guha’s work, and, in 2002, even sent a team to his IIT-Delhi lab. The team went back impressed with the concept but unimpressed with the way Guha and his team produced the drug in their lab.
An original drug developed in India is a feat of extreme rarity; one by an individual, rarer still. In Indian medical lore, just two such drug molecules have been credited to individual researchers: Dr Amiyo B Kar’s Centchroman, the world’s first non-steroid oral contraceptive (now marketed as Saheli), and Dr Upendranath Brahmachari’s Urea Stibamine, developed to treat Kala Azar. Professor Guha’s Risug could well be the third.
What sets Guha apart is that he has no medical qualification, though his PhD was in medical physiology and he even enrolled for an MBBS degree at the age of 39 (while already an IIT professor) to make up for that.
To work as a scientist in India requires not just the mind, but also a certain temperament. Sumana Das, a senior lab assistant with the Risug project at IIT-Kharagpur, calls it the ‘so what’ attitude. Das, who seems well trained in this attitude herself, says that in the seven years she has worked with Professor Guha, she has never seen him snap or fall ill. “So many permissions were due to come in, so much work could have been completed by now. At most, Sir keeps quiet for a couple of days. Then he starts humming again,” she says. The tune, she can’t quite place.
“It comes from both sides of my family,” says Guha. “My mother’s side were anarchists, my father’s side were conformists. Two of my uncles were imprisoned here at Hijli jail.” He refers to a British detention and torture centre on the premises of which IIT-Kharagpur was built. The old jail building now functions as an IIT museum and office, but Guha, in his time, once attended classes here. “One uncle spent eight years here, another about a couple of years,” he continues, “I get my insolence from there. My father had great respect for the establishment. I saw him destroy his health, looking after his patients and discharging all his official duties at Patna Medical College. But he’d never miss a day at work. That’s one reason I can keep going, because I saw him do that.”
The other reason Guha has been able to keep frustration at bay is that he has an abundance of ideas to chase. During the last decade alone, as Risug trundled along, Guha has worked on designing an artificial heart modelled on a cockroach’s heart. Now, this insect’s heart has 13 chambers in a series, while Guha’s model has two pumps, each with five chambers lined up one after another. The human heart has four chambers, but only two of these can pump blood. Besides, they are not in series. The great advantage of serial chambers is that even if a couple of them are unable to pump blood, the rest can pick up the slack. This is the reason, Guha says, cockroaches feature so prominently in science fiction: their hearts make them hardy enough to survive the most horrid doomsday scenarios.
This heart would be a bridge-to-transplant heart, which is an artificial heart that a patient is given till a usable donor heart is found. This usually costs Rs 60-70 lakh, while Guha’s model could be had for only Rs 1.5 lakh. At the moment, his team is awaiting permission to transplant this artificial heart into a goat on a trial basis.
It helps to have a wife like his: Mrs Guha has sat on dharnas outside Health Ministry offices with her husband, stays up till 4 am to eat dinner with him, and is even known to work in the lab alongside him to complete a model or drug solution when a presentation draws near.
His owlish ways extend to his fitness regime. Every night, at 3 am, the professor goes for a run on the campus. “When I run, my worries are nothing. Then I eat. I haven’t eaten breakfast and dinner on the same day for many years now. ”
We see him one night when we are there, this wiry man in a sharp pink T-Shirt, a muffler knotted stylishly around his neck, still a striking figure in his seventies. As Open’s photographer shuffles around for appropriate angles, he runs evenly, lightly, unaffected by the camera’s presence. Wrapped around his right hand is a leather belt to shoo dogs away. His breath is rhythmic, his gaze on the road steady. Steady, still, peaceful. Somehow.
This article was modified after it appeared in the print magazine
About The Author
CURRENT ISSUE
‘We Have Instilled Fear of the Law in Drug Syndicates,’ says Amit Shah
MOst Popular
4

/wp-content/uploads/2025/03/Cover_Amit-Shah.jpg)











More Columns
Elon Musk attracts sharp attack over ‘swastika’ from Indians on social media Ullekh NP
Yunus and the case of a "land locked" imagination Siddharth Singh
Why CSK Fans Are Angry With ‘Thala’ Dhoni Short Post